Biotechnology as a route to nanotechnology
By Ralph C. Merkle
www.ralphmerkle.com
Published in
Trends in Biotechnology,
July 1999,
Vol 17 No 7, pages 271-274.
This web version differs in some respects from the published version
and is available at http://www.ralphmerkle.com/papers/bionano.html
Abstract
It is common in manufacturing to hold, position, and assemble parts in a fashion that is not today possible at the molecular scale, yet the basic principles of positional assembly are just as applicable whether the parts are meters or nanometers in size. The ability to hold and position parts gives us remarkable flexibility in the manufacturing process, whether we are making furniture or synthesizing complex molecular structures. Applying this powerful concept at the molecular scale will require the development of new tools and pose new challenges in many fields, yet the rewards will be enormous.
Introduction
Nanotechnology is creating a growing sense of excitement because we see an opportunity of unprecedented magnitude looming on the horizon: the ability to arrange and rearrange molecular structures in most of the ways consistent with physical law. This will have a pervasive impact on how we manufacture almost everything -- what is manufacturing but a way to arrange atoms? If we can arrange atoms with greater precision, at lower cost, and with greater flexibility then almost all the familiar products in our world will be revolutionized. To name just three: we'll pack more computational power into a sugar cube than exists in the world today, we'll make inexpensive structural materials that are as light and strong as diamond (which will have a major impact on the aerospace industry), and we'll make surgical tools and instruments that are molecular in their size and precision, able to intervene directly at the fundamental level where most sickness and disease are caused.
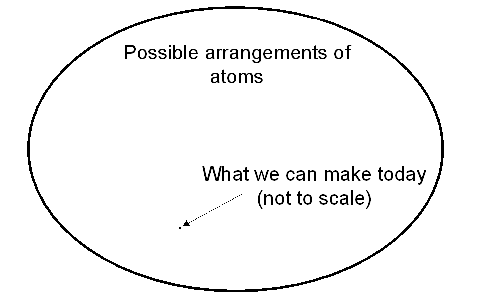 Underlying the excitement is a very simple fact: while atoms can be arranged in almost infinite permutations, today we can make only an infinitesimal fraction of what is possible. Very roughly, if we can pack 100 atoms into a cubic nanometer, and each atom can be any of the approximately 100 elements, then there are something like 100100 different ways we can arrange the atoms in just a single cubic nanometer. A cubic micron expands this to 100100000000000, while an object the size of you or me makes even this number seem vanishingly small. The goal that now seems possible: to take a healthy bite out of this enormous range of possibilities; to make most of the things that are possible, rather than an infinitesimally small fraction.
Underlying the excitement is a very simple fact: while atoms can be arranged in almost infinite permutations, today we can make only an infinitesimal fraction of what is possible. Very roughly, if we can pack 100 atoms into a cubic nanometer, and each atom can be any of the approximately 100 elements, then there are something like 100100 different ways we can arrange the atoms in just a single cubic nanometer. A cubic micron expands this to 100100000000000, while an object the size of you or me makes even this number seem vanishingly small. The goal that now seems possible: to take a healthy bite out of this enormous range of possibilities; to make most of the things that are possible, rather than an infinitesimally small fraction.
 In 1959 Feynman said: "The principles of physics, as far as I can see, do not speak against the possibility of maneuvering things atom by atom. It is not an attempt to violate any laws; it is something, in principle, that can be done; but in practice, it has not been done because we are too big." More recently, Smalley said "Most interesting structures that are at least substantial local minima on a potential energy surface can probably be made one way or another."
In 1959 Feynman said: "The principles of physics, as far as I can see, do not speak against the possibility of maneuvering things atom by atom. It is not an attempt to violate any laws; it is something, in principle, that can be done; but in practice, it has not been done because we are too big." More recently, Smalley said "Most interesting structures that are at least substantial local minima on a potential energy surface can probably be made one way or another."
The breathtaking magnitude of this opportunity is attracting interest. Neal Lane, the Director of NSF, said: "The possibilities of nanotechnology are endless. Entirely new classes of incredibly strong, extremely light and environmentally benign materials could be created" and went on to discuss inexpensive superconductors and medical applications. NSF is backing up this rhetoric with grants. NASA has a computational molecular nanotechnology research group examining the ways in which this technology can be used to advance the exploration and human habitation of space. IBM is doing pathbreaking research to revolutionize computing. Storing one bit in a few atoms no longer seems outlandish, and molecular switches will someday replace the bulky devices made today using optical lithography.
As we move beyond the vision and start asking how we are going to do this and how long it will take, opinions begin to diverge. Should we make ever better scanning probe microscopes (SPM's)? These remarkable instruments have already demonstrated an ability to move atoms and molecules on a surface in a controlled way (often spelling out names of interest to the researchers or their sponsors), but have so far been confined to two dimensions. Stacking molecules one on top of another is the next obvious goal, which will no doubt be accomplished in the next few years. Could these versatile instruments go on to make molecular machines?
Or perhaps the design and modification of proteins and their
self assembly will provide the key to progress?
Living systems already use many molecular machines,
such as molecular motors. Could we adapt them to our own uses,
perhaps using them to power tiny pumps or open and close
tiny valves?1
A computer generated image of a truncated
octahedron experimentally synthesized from DNA by
Nadrian Seeman.
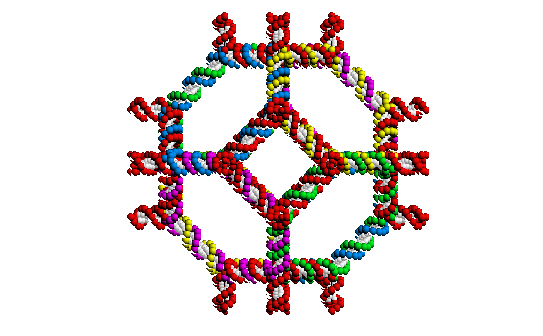 |
There are many novel uses of existing biopolymers that could provide us with new tools. DNA, for example, is known primarily for its ability to encode information. But it can also produce structures as complex as a truncated octahedron2 and even provide power when it's chemical conformation changes in response to changes in its environment3.
The great diversity of proposals, ideas, and experimental capabilities makes it very difficult to predict exactly how we will proceed towards the more general goals of nanotechnology. Yet there are a few principles that seem both powerful enough and clear enough that they can provide some sort of framework for orienting ourselves. The first principle we consider is that of positional assembly.
Positional assembly
There are two main ways to assemble parts. In self assembly, the parts move randomly under the influence of thermal noise and explore the space of possible mutual orientations. If some particular arrangement is more stable, then it will be preferred. Given sufficient time, this preferred arrangement will be adopted. For example, two complementary strands of DNA in solution will eventually find each other and stick together in a double-helical configuration.
In positional assembly, some restoring force keeps the part positioned at or near a particular location, and two parts are assembled when they are deliberately moved into close proximity and linked together. While common at the scale of humans (we commonly hold, position and assemble parts with our hands) this ability is still quite novel at the molecular scale. Thermal noise still plays a significant role, as "holding" a molecular part does not provide absolute certainty about its position but instead imposes a bias on the range of positions it can adopt. Using a linear approximation, an object might be subjected to a restoring force F which is proportional to its distance from the desired location, i.e., F = ks x, where x is the distance between the part and its desired location, and ks is the restoring force.
Restoring forces on the order of 10 N/m (Newtons/meter) or better can be achieved with scanning probe microscopes, which can position an object quite accurately. The fundamental equation relating positional uncertainty, temperature and stiffness is4:
s2
= kbT/ks
Where s is the mean error in position, kb is Boltzmann's constant, T is the temperature in Kelvins, and ks is the "spring constant" of the restoring force. If ks is 10 N/m, the positional uncertainty s at room temperature is ~0.02 nm (nanometers). This is accurate enough to permit alignment of molecular parts to within a fraction of an atomic diameter. It is important to remember, however, that the actual error could be many times s. The probability that the actual error is xerr is exp[-ks xerr2/(2s2)] / (s sqrt(2p)). Errors of a few times s are common, but errors of 20 times s would be extremely unlikely.
The distinction between self assembly and positional assembly is not binary, but moves continuously along a scale depending on the positional uncertainty (which is a function of the restoring force and the temperature). When the positional uncertainty s is large, we are near the self assembly end of the spectrum. When s is small, we are at the positional assembly end of the spectrum. Intermediate points along this spectrum are occupied by, for example, a molecule "tethered" to an SPM tip by a polymer; or an object held by optical tweezers (a restoring force of 10-4 N/m implies a positional uncertainty s at room temperature of ~6 nm).
While the SPM provides programmable positional control (you can adjust x, y and z to essentially any values), a simple form of positional assembly can also be seen in enzymes which bind two substrate molecules. The two bound molecules are positioned with respect to each other, thus facilitating their assembly. A limited form of positional assembly is also used in the ribosome, which can position the end of a growing protein adjacent to the next amino acid to be incorporated into that protein5.
This combination of positional assembly and self assembly can also be seen at the macroscopic scale. The vibratory bowl feeder6 is commonly used in manufacturing to position parts with sizes on the order of a centimeter. The bowl is shaken by a motor, causing parts in the bowl to bounce onto and along a spiral track leading out of the bowl. By careful design of the track, parts in the right orientation continue to move along it, out of the bowl and into further assembly steps. Parts in the wrong orientation are bounced back into the bowl, where they can try again to move up the spiral path leading out of the bowl.
While the power of self assembly has been amply demonstrated by the wide range of complex molecular structures it has made (including a remarkable range of biological structures), we have barely begun to explore the power of positional assembly at the molecular scale. Despite this, it seems clear that this new capability will play a major role in our future ability to synthesize molecular structures. The power of positional assembly has been amply demonstrated at the macroscopic scale in today's factories and by our own ability to make things with our hands. While its application at the molecular scale will differ in many details, it will provide a new and remarkably powerful tool for extending the range of structures that we can make.
Biotechnology and programmable positional control
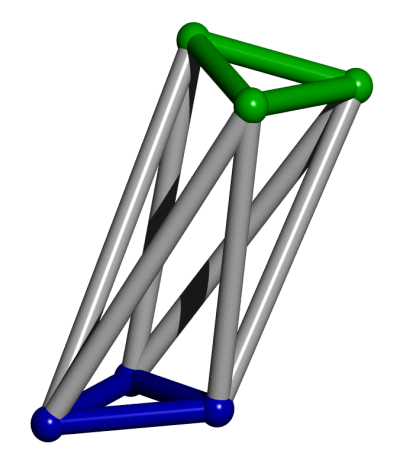
Schematic
illustration of a Stewart
platform. The lower (blue) triangle forms the base, while the
upper (green) triangle froms the platform.
The position and orientation of the platform with respect to the base
can be controlled in six degrees of freedom (X, Y, Z, rool, pitch, and
yaw) by adjusting the lengths of the six gray struts.
Today's SPMs are large, relatively slow,
and will never make mole quantities of product.
If we really want positional assembly to make products
in the volume that ribosomes make proteins,
we must have small, fast positional devices7.
Yet it seems unlikely that biotechnology will directly
give us a molecular robotic arm.
Which brings us to the Stewart platform8,9,10. This device, basically an octahedron six of whose struts can be lengthened or shortened under programmatic control (see illustration), provides six degree of freedom positional control for the "platform," (the green triangle at the top of the octahedron) with respect to its base (the blue triangle at the bottom of the octahedron). The ability to make an octahedron does not seem beyond the capabilities of biotechnology (in the broad sense of the term), particularly when the ability to self assemble a truncated octahedron has already been demonstrated by Seeman2.
All we need are twelve stiff struts, some way to make their ends stick together, and some way to lengthen or shorten six of those struts. As the latter seems the harder problem, we discuss one possible approach to solving it.
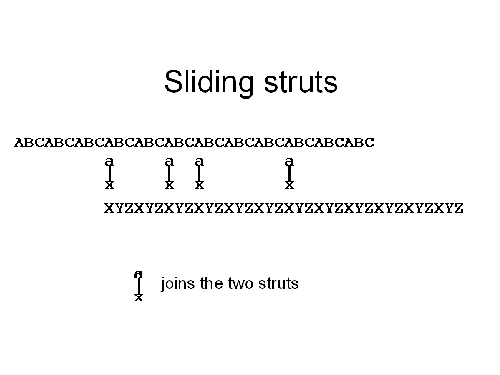
Consider a single strut: how can we change its length? One way would be to use two struts that overlap, and then make them slide past each other in a controlled fashion. Suppose that the first strut is made of three repeat units, ABCABCABCABCABC...., while the second strut is also made of three repeat units, XYZXYZXYZXYZ.... If we want to combine these two struts into one long strut, then we have to join them together. Suppose we use "joiners" that have two ends: one end binds to the A units of the first strut, while the other end binds to the X units of the second strut. Then, as illustrated above, the two struts would be held together by this a-x joiner to form a single longer strut.
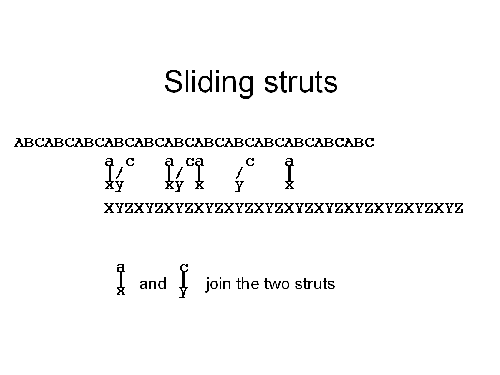
But how can we change the position of the ABC strut with respect to the XYZ strut? First, we add a c-y joiner. These new joiners will bridge between the C and Y units of the ABC and XYZ struts. They will at first be strained, but as we add more and more c-y joiners they will start to balance out the a-x joiners. If we now wash the a-x joiners out of solution (the simplest arrangement would be to anchor the octahedron in place by a tether, and flow a solution with an appropriate concentration of joiners past them), then the c-y joiners will dominate the linkage between the two struts leading to the results in the final illustration, below. At this point, the ABC and XYZ struts have moved past each other by one monomer.
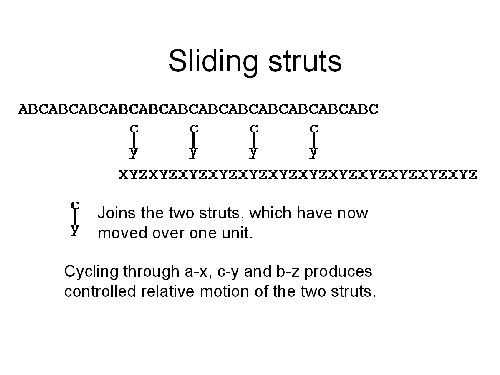
If we repeat the whole process again,
this time washing in a b-z joiner and washing out the
c-y joiner, we can again move the two struts over by one monomer.
Finally, if we wash in an a-x joiner and wash out the b-z joiner, we are back where we started. By repeating the whole cycle, we can move the ABC strut past the XYZ strut as much as we want. By running the cycle in reverse, we can reverse the motion. In essence, we have a three-phase linear motor. While this is slow (it's limited by the speed at which we can wash the joiners into and out of position) it does provide a flexible means of controlling the length of the strut, and does not seem hopelessly difficult.
The essential point here is not that this particular approach is the right one or even necessarily a good one, but that biotechnology and self assembly can be used to make positional devices. This is just one possible way: there are a great many more.
Building blocks
If we are to use positional assembly, we must have something to assemble. In biotechnology and self assembly, the most common building blocks are monomers that are built into polymers. Each monomer has two linkage groups which let it become part of a chain. The best known polymers are proteins, DNA, and RNA5. Proteins and RNA form complex three dimensional structures because the monomers from which they are made have strong preferences in how they bind to each other. By appropriately arranging the linear sequence of monomeric units, it is possible to indirectly control the three dimensional structure of the resulting polymer.
While the demonstrated capabilities of this approach are remarkable, if we could hold and position building blocks in three dimensions we should be able to assemble complex three dimensional structures much more directly from building blocks. To this end, we require building blocks that have more than two linking groups (as two linking groups would give us polymers, which provide only indirect control over three dimensional structure). Three points define a plain, and so three groups are frequently found in two-dimensional structures. In graphite, for example, each carbon atom is joined to three neighbors. Four points define a three-space, so four linking groups in tetrahedral symmetry are well suited to the formation of three dimensional structures, much as the carbon atoms in diamond are each bonded to four neighbors.
In the near term, building blocks that are relatively large (many atoms) and which can be readily manipulated in solution are more likely to be experimentally accessible. While synthetic methodologies and reactions for arranging carbon atoms in desired patterns have been proposed4,11,12,13, these approaches require very controlled conditions, extremely good absolute positioning capbabilities, and very clean ultra-high vacuum environments. While these conditions should be achievable in the future, at present they present formidable experimental challenges. Molecular building blocks made from many atoms can be designed that are easier to manipulate, easier to link, less susceptible to contaminants, and more easily positioned. We need to start with large, easy to use building blocks.
If we are to build large, stiff structures then the building blocks themselves should be stiff. Drexler has proposed the use of artificially designed proteins in which simultaneous use of many stabilization techniques produces a protein that is much more stable than naturally occuring proteins14. The recent explosion of interest in fullerenes has produced not only functionalized C60, which is both large and stiff, but also long fullerene tubes which can be functionalized at the ends. Adamantane, essentially a small fragment of diamond, is a tetrahedrally symmetric molecule with a 10-carbon framework which is very stiff. Over 20,000 substituted or functionalized variants of adamantane are known, and even more are possible.
The linking groups between building blocks should also be strong and stiff. Krummenacker15 proposed the use of dienes and dieneophiles as linking groups which would combine in a Diels-Alder cycloaddition. Because the diene and dienophile are selectively reactive, molecular building blocks with these groups would be relatively immune to contaminants and the reaction could take place in any of a wide range of solvents.
The requirements for building blocks designed for positional assembly are not the same as the requirements for building blocks that self assemble. For example, positionally assembled building blocks must not only link with each other, they must also bind and release from the positional device. Drexler proposed the use of antibody-derived proteins bound to an SPM tip to provide a flexible method of binding to and positioning a wide range of building blocks4.
A second difference is that the linkage groups between positionally assembled building blocks can be much stronger. In self assembly, the use of many weak bonds (such as hydrogen bonds) provides a high degree of specificity in the inter-building-block reactions5. More reactive moieties (radicals, for example) would tend to combine promiscously, and so cause the self assembled building blocks to "clump" in random patterns rather than forming the desired structures. By contrast, positionally controlled building blocks can be more reactive, provided that encounters between building blocks are controlled. Rather than stirring positionally controlled building blocks together in solution, they could be introduced while bound to a surface. The positional device would pick them up from the surface and then move them to a work area where they would be joined to other building blocks which had already been formed into a partial structure. At no point would the positionally assembled building blocks be allowed to encounter each other in uncontrolled or random orientations, thus eliminating undesired side reactions.
Conclusions
While there is general agreement that we will, in the not too distant future, be able to arrange atoms in most of the patterns consistent with the laws of physics, there is at present no general consensus about how best to achieve this objective. Because the range of possible approaches is so great, it is unclear which specific approaches are the best candidates for further research. One approach that seems particularly powerful is to combine our existing arsenal of synthetic methods with novel (at least at the molecular scale) approaches that position the reacting species where we want them to provide greater selectivity over their interactions and to suppress undesired side reactions.
References
- Montemagno, C., Bachand G., Stelick S., and Bachand, M. (1998) Sixth Foresight Conference on Molecular Nanotechnology, http://www.foresight.org/Conferences/MNT6/Papers/Montemagno/index.html.
Constructing Biological Motor Powered Nanomechanical Devices
- Zhang, Y., and Seeman, N.C. (1994) Journal of the American Chemical Society 116, 1661-1669.
The Construction of a DNA Truncated Octahedron
- Mao, C., Sun, W., Shen, Z., and
Seeman, N.C.
(1999) Nature 397, 144-146. A DNA Nanomechanical Device Based on the B-Z Transition
- Drexler, K.E., (1992)
Nanosystems: Molecular Machinery, Manufacturing, and Computation,
Wiley-Interscience
- Watson, J.D., Hopkins, N.H., Roberts, J.W., Steitz, J. A., Weiner, A.M. (1987) Molecular Biology of the Gene, Fourth Edition, Benjamin/Cummings..
- Riley, F.J. (1983) Assembly Automation, Industrial Press.
- Merkle, R.C. (1997) Nanotechnology 8, 47-52.
A new family of six degree of freedom positional devices
- Stewart, D (1965-66) The Institution of Mechanical Engineers, Proceedings 1965-66, 180 Part 1, No. 15, pp. 371- 386. A Platform with Six Degrees of Freedom
- Gough, V.E., and Whitehall, S.G. (1962) Proc. 9th Int. Tech Congress F.I.S.I.T.A., pp. 117. Universal Tyre Test Machine
- Fitzgerald, J.M. and Lewis, F.L. (1993) Robotics Today, 6, pp. 1-3. Evaluating the Stewart Platform for Manufacturing
- Musgrave, C. B., Perry, J.K., Merkle, R.C. and Goddard W.A. (1991) Nanotechnology 2, 187-195.
Theoretical studies of a hydrogen abstraction tool for nanotechnology
- S.P. Walch, W.A. Goddard, and R.C. Merkle. Fifth Foresight Conference on Molecular Nanotechnology (1997).
Theoretical studies of reactions on diamond surfaces
- Sinnott, S.B., Colton, R.J., White, C.T., and Brenner, D.W. (1994) Surface Science 316 (1994) L1055-L1060. Surface patterning by atomically-controlled chemical forces: molecular dynamics simulations
- Drexler, K. E. (1994) Annual Review of Biophysics and Biomolecular Structure (23:337-405). Molecular machines: physical principles and implementation strategies
- Krummenacker, M., (1994) Chem. Design Autom. News, 9, p. 1 and 29-39. Steps
Towards Molecular Manufacturing
 Underlying the excitement is a very simple fact: while atoms can be arranged in almost infinite permutations, today we can make only an infinitesimal fraction of what is possible. Very roughly, if we can pack 100 atoms into a cubic nanometer, and each atom can be any of the approximately 100 elements, then there are something like 100100 different ways we can arrange the atoms in just a single cubic nanometer. A cubic micron expands this to 100100000000000, while an object the size of you or me makes even this number seem vanishingly small. The goal that now seems possible: to take a healthy bite out of this enormous range of possibilities; to make most of the things that are possible, rather than an infinitesimally small fraction.
Underlying the excitement is a very simple fact: while atoms can be arranged in almost infinite permutations, today we can make only an infinitesimal fraction of what is possible. Very roughly, if we can pack 100 atoms into a cubic nanometer, and each atom can be any of the approximately 100 elements, then there are something like 100100 different ways we can arrange the atoms in just a single cubic nanometer. A cubic micron expands this to 100100000000000, while an object the size of you or me makes even this number seem vanishingly small. The goal that now seems possible: to take a healthy bite out of this enormous range of possibilities; to make most of the things that are possible, rather than an infinitesimally small fraction. In 1959 Feynman said: "The principles of physics, as far as I can see, do not speak against the possibility of maneuvering things atom by atom. It is not an attempt to violate any laws; it is something, in principle, that can be done; but in practice, it has not been done because we are too big." More recently, Smalley said "Most interesting structures that are at least substantial local minima on a potential energy surface can probably be made one way or another."
In 1959 Feynman said: "The principles of physics, as far as I can see, do not speak against the possibility of maneuvering things atom by atom. It is not an attempt to violate any laws; it is something, in principle, that can be done; but in practice, it has not been done because we are too big." More recently, Smalley said "Most interesting structures that are at least substantial local minima on a potential energy surface can probably be made one way or another."